8 Hacks to Nail Your Next Plain Language Summary
16 November, 2021
By Julie Henderson and T. DeLene Beeland
A plain language summary (PLS)—also known as a lay summary or trial results summary—is a piece of writing that briefly explains the who, what, when, where, why, and how of clinical trials.
Pharmaceutical and biotech companies publish the results of their clinical trials in peer-reviewed journals. But patients don’t usually read these, and there is an increasing demand from the general public to access trial findings in everyday normal language.
People who participate in medical research have a right to know what a trial seeks to do, what their part in that journey is, and what results were discovered. So when writing a PLS, your job is to craft an easy-to-understand summary of the trial’s aims and results in a way that participants and the interested public can digest without diving for a dictionary.
The European Union has taken note of consumer interest for easy-to-understand information about clinical trials. It plans to implement a new regulation this winter (2021/22). Known as the EU Clinical Trials Regulation (CTR) 536/2014, it will require sponsors of trials conducted in EU countries to publish summaries of their studies and findings in language understandable by non-experts. Which is where plain language enters the picture.
Writing in plain language means that the “wording, structure, and design are so clear that the intended audience can easily find what they need, understand what they find, and use that information,” according to the International Plain Language Federation. Crafting your PLS to this standard requires imagination and planning, in equal measures.
It’s important to get a PLS right. When done well, these summaries can improve health literacy in the general public and encourage the pharmaceutical companies that sponsor clinical research to operate more transparently.
These eight hacks, adapted from tips by the Plain Language Association International and The Center for Plain Language, will help you master your next PLS.
1. Who Will Read Your Plain Language Summary?
Before you get started, identify your audience. Plain language summaries are written for the people who participate in clinical trials and the general public. Assume that your readers are unfamiliar with medical, pharmaceutical, and research jargon, which means you need to meet them on their reading level and adopt less technical terms to impart core content.
Start with a quick mental exercise. Imagine talking about your PLS to a friend or relative who works outside of the spheres of research or medicine. How might the conversation sound? This is where you want to aim your writing approach. A PLS must be understandable to a layperson, which equates to a sixth to eighth grade literacy level in the United States. Keep in mind, your audience is likely smart but may not have a high level of health literacy. (See hack #4 for readability tools to help you write at the target level!)
2. Get to Know the Template Structure
You won’t need to invent the wheel when you begin your PLS; the new regulation provides a helpful template. If the research is completed, then gathering the material to include in the template is easy. There are no page or word counts for a PLS, but most are five to six pages long. (That said, please respect your reader’s patience; shorter is best.) However, the new regulation does require each PLS to contain 10 core elements which can easily form your structural organization. These elements are named in bold in the table below and should be retained word-for-word in the PLS. In fact, they make great level-1 headings. (See the EU Commission PLS guidelines document for more details.)
|
EU CTR 536-2014 PLS Requirement |
|
Plain Language Template |
|
1. Clinical trial identification (including title of the trial, protocol number, EU trial number and other identifiers) 2. Name and contact of sponsor |
Page 1 (title page) In addition to the required information, indicate the name and, in nontechnical terms, the type of drug or therapy that was studied. Many sponsors also include a thank-you to participants on the title page. |
|
|
3. General information about the clinical trial (including where and when the trial was conducted, the main objectives of the trial, and an explanation of the reasons for conducting it) |
Why was this clinical trial done? What were the goals of the trial? What kind of study was this?a Where and when was the study conducted? |
|
|
4. Population of subjects (including information on the number of subjects included in the trial in the Member State concerned, in the Union, and in third countries; age group breakdown and gender breakdown; inclusion and exclusion criteria) |
Who took part in this trial? How were people selected to be in the trial? What were their characteristics? |
|
|
5. Investigational medicinal products used |
|
(See page 1) |
|
6. Description of adverse reactions and their frequency 7. Overall results of the clinical trial 8. Comments on the outcome of the clinical trial 9. Indication if follow-up clinical trials are foreseen |
What happened during the study? What were the results of this clinical trial? Did participants have any adverse reactions?b If so, were any reactions serious? |
|
|
10. Indication where additional information could be found |
Where can I learn more about this clinical trial? |
aFor example, dose ranging, safety and efficacy, placebo controlled: name and explain in plain language.
bRather than “side effects,” the terms “adverse reactions” or “adverse events” are preferred by many trial sponsors and in the new regulations.
3. Ready, Set, Write (in Plain Language)
Writing in an approachable, conversational style can be the most creative and challenging part of your PLS. It can also be the most rewarding. Remember, plain language is what unlocks all the useful and interesting data from a clinical trial and makes it accessible to wider audiences—and you hold the keys.
Words and tone matter. In general, shorter sentences with simpler terms will strategically target your reader’s literacy level. A few tips:
- Address your reader as “you.”
- Keep sentences and paragraphs short and to the point. (Pro tip: spot check your sentences; the average length should be fewer than 15 words.)
- Be lively and interesting, use the active voice where possible.
- Choose words with fewer syllables (e.g., “use” instead of “utilize”).
- Select common terms over technical ones (e.g., “itchy” instead of “pruritic”; “heart” over “cardiac”).
Numbers matter too. When you must use numbers, keep the data simple and easy to understand. Percentages are straightforward; odds ratios and P values are not. If possible, paint a picture for your reader with your words. It may be hard for someone to understand what “three-quarters of participants” refers to, but “three in four participants” makes intuitive sense.
You needn’t ditch technical terms. When you must use important technical terms, employ the “name and explain” technique. Introduce the term, then explain it using easily understood words. This can add to the word count, but don’t despair. Sometimes a plain language explanation takes at least twice as many words to get the same idea across—and that’s okay. Very few laypeople would know what a “nosocomial infection” is. However, they’d be sure to understand if you said it was an “infection you get while in the hospital.” You’re using seven words in this phrasing, instead of two—but the meaning is crystal clear instead of opaque.
Too many technical terms? Try a glossary. It can be helpful to provide readers with a glossary that defines the meaning of medical terms used in the PLS, and it can be helpful to use parenthetical explanations. For example, you could say that “the new drug is intended to reduce urticaria (itchy rashes) in people who have eczema.” This method shares the technical term with the reader while helping them understand its meaning.
Abbreviations. Abbreviations should be used with a light touch. No one wants to read about “H&Ps that measure TPR, BP, sx, and I&O.” However, some abbreviations are more familiar to readers than the expanded term. For example, you’re likely more used to saying “CPR” than “cardiopulmonary resuscitation,” and more used to seeing “HIV” than “human immunodeficiency virus.” (Pro tip: If you’re unsure, try reading your work aloud. Anything that doesn’t sound conversational will immediately stand out.)
4. Check Out a Reading Scale
Readability scales are tools that can help hone your PLS to the targeted audience. These scales—which include the Flesch Reading Ease, Flesch–Kincaid Grade Level, Fry Readability Test 3, SMOG (Simplified Measure of Gobbledygook test), and Lexile Framework for Reading—use formulas that incorporate word length, syllables, sentence length, the number of sentences per paragraph, and other factors to arrive at a readability score or grade.
The Flesch Reading Ease and Flesch–Kincaid Grade Level formulas are already loaded into Microsoft Word. To activate these tests, under options for spelling and grammar, check the box that says “Show readability statistics." Afterward, every time you run spellcheck on your document, readability scores will pop up. Again, your goal is a sixth to eighth grade level.
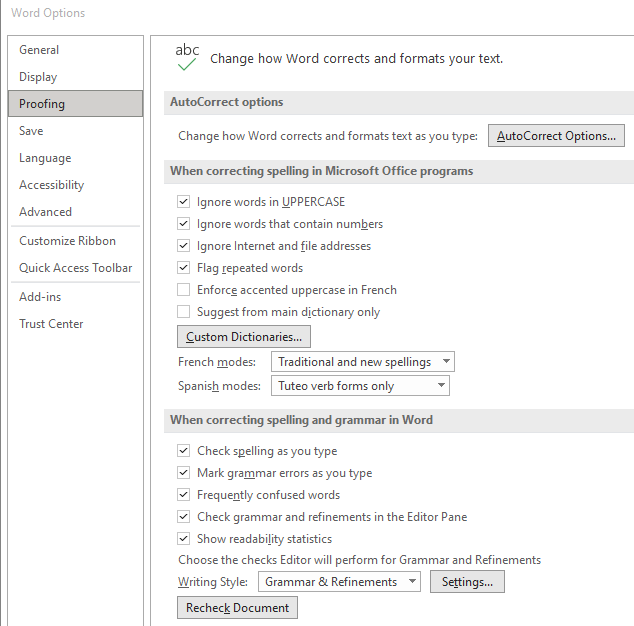 Microsoft Word Readability Statistic Setting (Enlarge image)
Microsoft Word Readability Statistic Setting (Enlarge image)5. Report the Objective Facts
Put on your reporter hat and stick to the facts; a PLS is a promotion-free zone. When writing, you will want to emphasize the objective, informational nature of the summary and avoid any perception of promoting a specific trial drug or therapy, or even of judging the trial findings.
For example, the statement “drug A outperformed drug B in preventing asthma exacerbations” could be phrased “in this trial, patients who took drug A had fewer asthma attacks than those who took drug B.” Reporting on the facts of the specific trial, using neutral language and a balanced approach will help avoid misperception about the trial results. This means you should avoid superlatives or talking up one trial therapy more than another.
Readers should also not be led to think that the findings outlined in the PLS apply to more than just the one trial being reported. That is, after reading the PLS, a reader shouldn’t think the trial results are definitive with regard to the investigative drug. They should understand that the findings come from one single study.
6. Design for Readability
Design elements can help guide the reader through the PLS and aid their reading comprehension. Text size and font style should be chosen carefully; avoid text that’s smaller than 12 points, and choose standard fonts rather than fancy ones.
Use creative ways to present information more visually. Readers enjoy a little white space, and bulleted lists, diagrams, images, and tables may impart concepts better than long paragraphs. Tables and diagrams should be uncluttered and present easily understandable data. Color can be used to enliven the visual appearance of PLS, but colors that bring to mind specific corporate brands and company logos should be avoided.
7. Seek Feedback. (Then Use It.)
Proactively seek out feedback from a lay person, such as a trial participant, or a focus group. Ask them to read the draft PLS and then tell you what they learned.
Does their understanding match what you meant to convey? If not, their “incorrect” answers can guide you on where and how to clarity your PLS.
8. Let Readers Know What to Do Next
The last element required in your PLS by the new regulations is a statement about where more information about the trial can be found. This may be the Clinical Trials Information System, the EU Clinical Trial Register, or a corporate website. Trial sponsors may also want to add boilerplate text cautioning participants or readers not to change their medication or health regimen on the basis of the results of a single trial without first talking to their health care provider.
Creating a Plain Language Summary? PerfectIt Can Help
You probably figured out by now that there’s a lot to remember when creating a PLS. One thing that can make life easier is consistency software like PerfectIt™ for Microsoft Word.
Running PerfectIt will instantly spot inconsistencies in capitalization, hyphenation, punctuation, and numbering in your PLS. It will also find undefined acronyms, as well as acronyms that you accidentally defined more than once. And that glossary you want to include in your PLS? PerfectIt will generate a list of all the acronyms you’ve used, along with their definitions.
You can also easily customize PerfectIt to suggest plain language alternatives for medical jargon. PerfectIt can recommend that you use "nerve pain" instead of "neuralgia," for example, or "inflammation of the kidneys" instead of "nephritis."
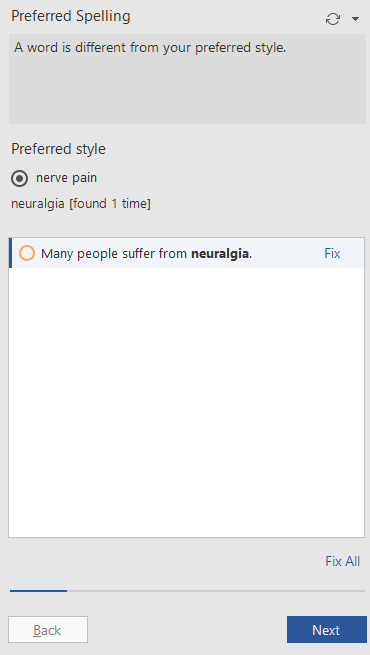 Preferred Style in PerfectIt 5 (Enlarge image)
Preferred Style in PerfectIt 5 (Enlarge image)Plain language summaries are essential. PerfectIt can be an indispensable writing companion that helps you create these summaries quickly and easily.
Julie Henderson, MS, RN, BELS, is a science and medical editor at Dragonfly Editorial, an agency that helps companies worldwide create clear, powerful content.
DeLene Beeland is a freelance medical and science writer who often writes for Dragonfly.




